 What is Anthracene?
What is Anthracene?
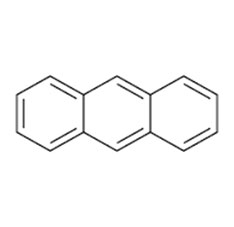
Anthracene is a solid polycyclic aromatic hydrocarbon of formula C₁₄H₁₀, consisting of three fused benzene rings. It is a component of coal tar.Anthracene is colorless but exhibits a blue fluorescence under ultraviolet radiation.
Uses
Anthracene is used in the production of the red dye alizarin and other dyes.
Sources & Potential Exposure
Workers who process steel and asphalt and roofing products or where combustion processes are extensive may breathe in anthracene from the air or have direct skin contact. The general population may be exposed by breathing in tobacco smoke, automobile exhaust or paving fumes. The general population may also be exposed to anthracene when eating grilled and smoked meat as well as when eating food prepared with some plant oils. If anthracene is released to the environment, it will be broken down in air. Anthracene released to air will be in or on particles that eventually fall to the ground. It is expected to be broken down by sunlight under certain conditions. It will not move into air from moist soil and water surfaces. It is expected to move slowly through soil. It will be broken down by previously exposed microorganisms and is expected to build up in fish. Data on the potential for anthracene to produce toxic effects in humans were limited to a single study, which reported anecdotal evidence of a potential link between prolonged use of anthracene-containing oral laxatives and increased incidence of black pigments in the lining of the colon and rectum (melanosis)
Federal Regulations
EPA: Confirmed human carcinogen.

 Americas
Americas Europe
Europe Français
Français Deutsch
Deutsch Italiano
Italiano Español
Español