 What is TCA?
What is TCA?
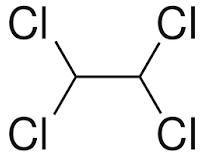
Tetrachloroethane is a chlorinated derivative of ethane. It has the highest solvent power of any chlorinated hydrocarbon.
Uses
The production of tetrachloroethane as an end-product has decreased significantly in the United States. In the past, tetrachloroethane was used in large amounts to produce trichloroethylene, tetrachloroethylene, and dichloroethylene. It was also used as a solvent, in cleaning and degreasing metals, in paint removers, varnishes and lacquers, in photographic films, as an extractant for oils and fats, and in pesticides.
Sources & Potential Exposure
As tetrachloroethane is no longer used much in the United States, current air emissions predominantly result from its use as a chemical intermediate during the manufacture of other chemicals. Low levels have been detected in air. The main effects of tetrachloroethane are liver and neurological effects. Acute (short-term) inhalation exposure to very high levels of tetrachloroethane has resulted in effects on the liver and respiratory, central nervous, and gastrointestinal systems in humans. Chronic (long-term) inhalation exposure to tetrachloroethane in humans results in jaundice and an enlarged liver, headaches, tremors, dizziness, numbness, and drowsiness. Animal studies have shown a significantly increased incidence of liver tumors in mice orally exposed to tetrachloroethane. Tetrachloroethane has been detected in surface water and groundwater; however, a nationwide survey of drinking water supplies in the 1980s did not find any supplies containing tetrachloroethane.
Federal Regulations
EPA has classified tetrachloroethane as a Group C possible human carcinogen.

 Americas
Americas Europe
Europe Français
Français Deutsch
Deutsch Italiano
Italiano Español
Español